Ten Things to Look Out for While Reporting Under the Sunshine Act
The Physician Payments Sunshine Act became law as a part of the Affordable Care Act. This act was established with the aim of improving transparency among patients about the financial interactions between life science organizations and Healthcare Professionals (HCPs). The Sunshine report requires distributors and manufacturers of drugs/devices/supplies to report payments and other transfer of value made to physicians and teaching hospitals. 2021 ushers in new changes to the data collected which includes the addition of Non-Physician Practitioners. This adds the burden on representatives who report data. Gridlex provides tools including the Sunshine Act Reporting Management App that aims to make life easy for reporting entities.
Here are Ten Things Reporting Organizations and Physicians Need to be Aware of.
The definition of a “covered recipient” will be expanded to include five more provider types. Namely,
Additionally, the “nature of payment” category will also be updated to include three new categories. Namely,
The most prominent highlight of these changes includes the addition of Non-Physician Practitioners (NPP) as recipients who have to be included while reporting.
The Consumer Price Index is what determines the threshold for reporting. This figure changes every year. From January to December 2020, the threshold for payments/transfer of value was $10.97 and an aggregate of $109.69. The figures for the year 2021 are $11.04 and $110.40. This means that reporting entities do not have to report payments/transfer of value if it is lesser than $11.04. Similarly, if the aggregate amount of payments/transfer of value to a physician/teaching hospital in a year is less than $110.40, it need not be reported.
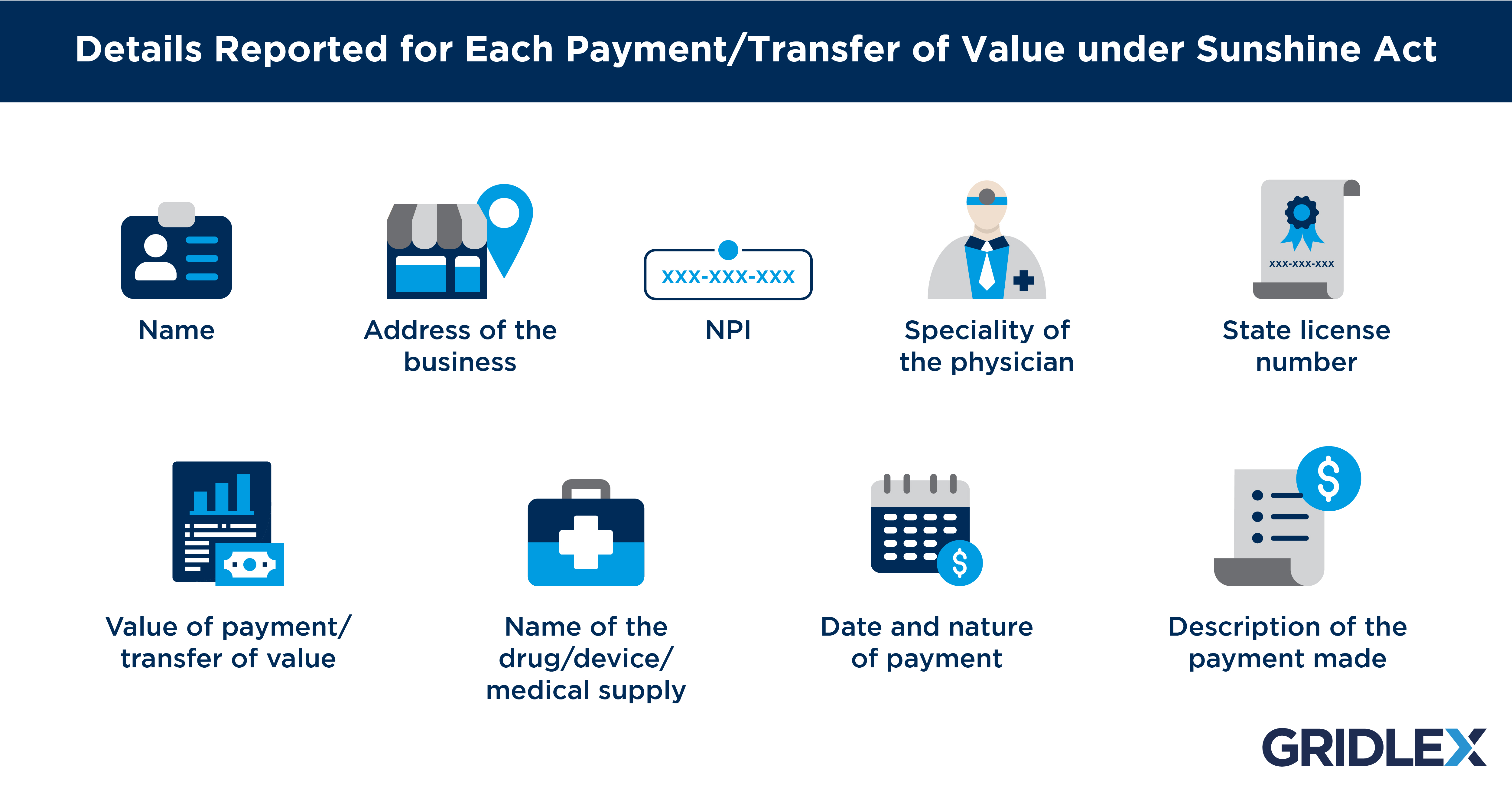
Reporting entities must also be aware of specific details that are collected under this act. This includes the name of the recipient, the specialty of the physician, state license number, name of the medical device/drug/medical supply, and description of the payment made.
Life Science Organizations conduct Continuing Medical Education (CME) to market their drugs and devices. Under the Sunshine Act, these expenses also need to be reported. PhRMA’s code of interaction with HCPs stresses three main points in relation to meals provided during CMEs
Tracking the names of physicians who partake in a meal at a CME event can be a difficult task. This is why reporting is not mandatory under such circumstances. But, meals such as company-sponsored dinners involve a transfer of value and should be reported under the Act.
Here are a few examples of how Sunshine act reporting works for meals:
Earlier: If a meal worth $220 is consumed by 5 physicians and 5 non-physicians, the reporting entity would report ($22X5) $110 under the Sunshine Act.
Sunshine Act Reporting Requirements 2021: If a meal worth $220 is consumed by 5 physicians and 5 non-physicians, the reporting entity should report ($22X10) $220 under the Sunshine Act since non-physicians are included in the reporting process from 2021.
Life science organizations distribute medical journals to HCPs. This is done to educate them about the organization’s products and services. Payments that need to be reported by reporting entities include medical textbooks and peer-reviewed journals. While reporting this to CMS, organizations should clarify all assumptions that were made to calculate the value of reprints. CMS states that “the value of a reprint should reflect the cost that the manufacturer paid to acquire the reprint from the publisher.”
One of the many challenges when it comes to publications is that reporting entities have to keep track of how many medical journals/reprints are being used for internal purposes and how many to be distributed to physicians. The bottom line is that under the Sunshine Act, reprints have to be reported if they have a value that exceeds $10/reprint and if the aggregate spending on it exceeds $100.
CME conducted by different reporting entities invites physicians as speakers at the event. The Sunshine Act Report has made it clear that the payment made to the speakers at such industry-supported continuing education events, need not be reported under the act.
If the event is accredited/certified by organizations like the ACCME, then the reporting entity plays no role in choosing or suggesting a speaker.
An indirect payment includes payments/transfer of value made through a third-party. Physicians or hospitals may at times request the manufacturer to make the payment to a third party. All these payments need to be reported under indirect payments. The only exception to this requirement is if the reporting entity is completely unaware of the identity of the third party.
An example of such an exception would be payments made to a third party for double-blind market research. Since anonymity is an important prerequisite, such payments are not included in indirect payments under the Sunshine Act. Representatives must inform doctors while distributing reprints that they will be reported under the act.
The act does not deem it necessary for organizations to report materials that are published specifically to benefit patients. If the reporting entity hands over or transfers a book, anatomical, or wall model to the physician, in order to interact with/educate the patient, then it need not be reported since it benefits the patient directly.
In contrast, if the reporting entity transfers books, materials, or journals for the benefit of the physician’s education or use, then it needs to be reported since it does not benefit patients in any manner.
Reporting entities must also submit details related to payments made on research. In some cases CMS permits a delay in reporting if the research is related to a) R&D of a new drug/medical device or a new application of an existing drug/medical device. b) Clinical investigation in relation to a new drug/device.
The reporting entity must first indicate if the payment for research is eligible for delayed reporting. Following this, the organization must annually mention the area towards which the payment is related, in its report. This could include FDA approval or licensure.
The report must include payments made in whole or part towards research to a covered recipient. This will also include the name of the research institution/individual, NPI, license number, specialty, and the recipient’s business address. On the other hand, payments for Business Development Plans will not be given any extension of time by the CMS.
-
Changes in Data Collection in 2021:
-
Nurse practitioners
-
Clinical nurse specialists
-
Physician assistants
-
Certified nurse-midwives
-
Certified registered nurse anesthetists
-
Long term medical supply or device loan.
-
Acquisitions
-
Debt forgiveness
-
-
Change in Reporting Thresholds
-
Details Collected Include NPI & State License Number
-
Expenditure on Meals and CME are Reportable:
-
The meal should be modest as judged by local standards.
-
Should not be a part of entertainment or recreational activities.
-
Should not take precedence over informational communication.
-
-
Reprints are Reportable
-
Compensation for Speakers is not Reportable
-
Exceptions in Third-party Vendors
-
Patient Education Materials are Not Reportable
-
Report and Review:
-
The reporting entity must be registered with the Enterprise Identity Management System (EIDM) and the Open Payments System. According to the CMS, this two-step process is done to prevent people from using an identity fraudulently.
-
All reporting entities must submit their reports by the 31st of March. Data can be reported by entering it manually or by uploading files.
-
After the submission of the final data, the submitted data is verified with teaching hospitals and physicians.
-
If the data does not match, reporting entities are given 15 days to rectify and resolve disputes and errors. It is essential to note that the report is not considered final until this attestation process is complete.
-
-
Reporting on Research Expenditure:
Gridlex's Sunshine Act Reporting Management App
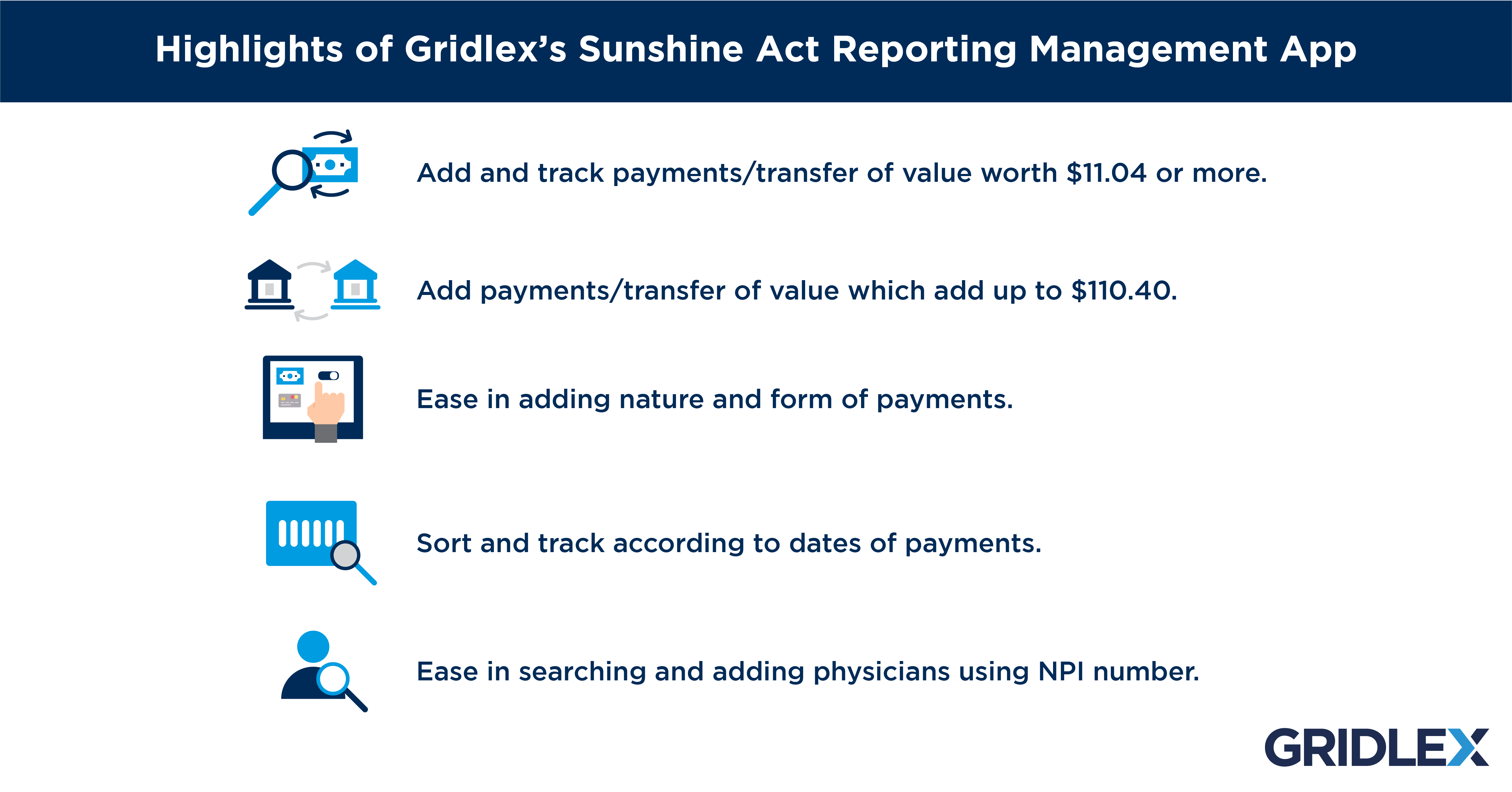
The Sunshine Act Reporting App by Gridlex will assist reporting entities in preparing themselves for the expansion of the Sunshine Act. The application can be customized to suit the specific needs of reporting entities. To ease this transition into the new guidelines, Gridlex’s application provides features that include tools for easy reporting, tracking payments in one place, and managing aggregate spending according to the reporting thresholds.
Gridlex’s Sunshine Act Reporting Management App aims at simplifying the process of reporting payment data to CMS. Collecting and keeping track of all payments/transfer of value made to physicians can be quite a hassle. The application helps reporting GPOs and manufacturers keep track of expenses which are to be reported to the CMS under the Sunshine Act.
Sunshine Act Reporting Management app is just one of the many features of Gridlex’s CRM that include features such as MIRF Management and Marketing Automation.
